YHLO Contributes to Lancet SARS-CoV-2 NAb Research
The mixed-and-match vaccine against Covid-19 tested a stronger immune response than two doses of AstraZeneca vaccine.
The chief scientist of the World Health Organization advises individuals not to mix and match COVID-19 vaccines from different manufacturers. So far, there is no available data to show how well the human body responds to this mixed-and-match vaccine and begins to form antibodies.
Researchers at the Technical University of Munich (TUM), Helmholtz Zentrum München, Universitätsklinikum Erlangen and Universitätsklinikum Köln are now studying this immune response within the framework of a retrospective scientific research.
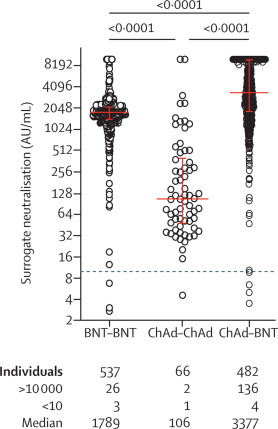
The blood samples came from 500 people who received the second BioNTech/Pfizer mRNA vaccine nine weeks after the first AstraZeneca vaccination.Using YHLO's NAb kit to test these blood samples, the results showed that these people had a much higher neutralizing antibody response than those who received two doses of the AstraZeneca vaccine. It has been shown that the immune response to the mixed-match vaccine is as good as the antibody response after two vaccinations with BioNTech/Pfizer's mRNA vaccine. The research has now been published in the journal The Lancet Infectious Diseases (Impact Factor: 25.071).
This study used chemiluminescence SARS-CoV-2 NAb kits produced by Shenzhen YHLO Biotech Co., Ltd to detect the neutralizing antibodies after different vaccination schedules. At the same time, this study also conducted a rigorous performance evaluation of the YHLO chemiluminescence method for SARS-CoV-2 NAb, which was highly recognized and unanimously praised by the research team.
*Original publication:
Tenbusch et al., 2021: Heterologous prime-boost vaccination with ChAdOx1 nCoV-19 and BNT162b2. The Lancet Infectious Diseases, DOI: 10.1016 / S1473-3099 (21) 00420-5
#Vaccines #Diagnostics #Serology #COVID19 #SARSCoV2 #Coronavirus #NeutralizingAntibodies #NAb #Covid19testing #Covid19solutions
Attachments:
Previous
Previous Page
MORE NEWS
YHLO Secures Approval for the World’s First sCD146 CLIA Assay
YHLO has obtained approval from the Guangdong Medical Products Administration for its soluble CD146 (sCD146) CLIA assay, marking a major milestone in central nervous system (CNS) disease diagnostics. YHLO is now the first company worldwide to offer an sCD146 test for cerebrospinal fluid, bringing its total CLIA assay portfolio in China to 173.
2025-11-18
YHLO Showcases New Products at the 2025 ADLM Exhibition
This year, YHLO made its international debut at the event, showcasing new products at the ADLM 2025.
2025-08-04
Add: Building 1, YHLO Biopark, Baolong 2nd Road, Baolong Subdistrict, Longgang District, 518116 Shenzhen, P.R.China







